Sulphuric Acid Strong or Weak
As a result the aqueous solution is pH neutral. Sulphuric acid carefully along the sides of the test tube.

Ph Of Lactic Acid Strong Or Weak Techiescientist
The salt strontium sulfate is formed with water as a product of the reaction between sulphuric acid and strontium hydroxide.

. Strontium hydroxide is a base. Hydrogen gas can be a problem with carbon steel in sulphuric acid environments because it can literally scrub off the mechanically weak iron sulphate film which is the only thing protecting the steel from attack. A weak acid in other terms is an acid that is not strong.
654 SMo UNS S32654. Since that time new data have become available which have been incorporated in this Monograph and taken into consideration in the present evaluation. It is this film of corrosion product that once formed prevents further corrosion of the underlying material.
Type 310M Corrosion Rates in Strong Sulphuric Acid. Explain the role of water in dissociation of acids and bases. It is a strong acid.
Pure water is a weak acid as well as a weak basic. Differentiate between strong and weak acids and bases. Carbon steel in the presence of strong sulphuric acid will corrode to form a thin film of iron sulphate on the surface of the metal.
Hydrochloric acid and strontium hydroxide. Sulphuric acid dissociates completely in an aqueous solution and produces H 2 and SO 4 2-. Isopropyl alcohol and isopropyl alcohol manufacture strong-acid process were considered by previous IARC Working Groups in 1977 and 1987 IARC 1977 1987.
Weak acids are acids that do not dissociate entirely in solution. H 2 C 2 O 4 CH 3 COOH lactic acid phosphoric acid acetic acid or vinegar etc. A separate Monograph on Mists from Strong.
This is because a little amount of water dissociates into protons and hydroxide anions forming hydronium. Isocorrosion Curves Corrosion Rate 01 mmy 4 mpy. Bring a burning match stick near the mouth of.
Are a few examples of weak acids. As well the formation of weak acid in the system may lead to increased corrosion and eventual leaks. The strength of weak acid is measured by the amount of dislocation in it-the more it dissociates the stronger the acid becomes.
Set the apparatus as shown in the Fig. Aqueous sodium sulphate solution is hydrolyzed to create sodium hydroxide and sulphuric acid both of which are strong bases and acids.

Is H2so4 An Acid Or Base Strong Vs Weak Sulfuric Acid

Is H2so4 An Acid Or Base Strong Vs Weak Sulfuric Acid

Is H2so4 An Acid Or Base Strong Vs Weak Sulfuric Acid
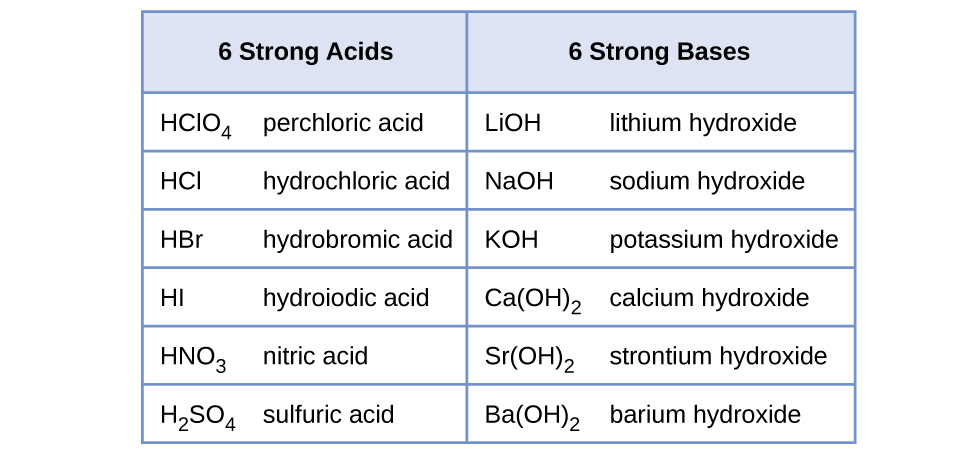
14 3 Relative Strengths Of Acids And Bases Chemistry 112 Chapters 12 17 Of Openstax General Chemistry
0 Response to "Sulphuric Acid Strong or Weak"
Post a Comment